The Ultimate Guide to Crystalline vs. Amorphous Polymers
Crystalline polymers are characterized by their neatly packed and organized molecular structures, which come with specific melting points (Tm). On the other hand, amorphous polymers have a more chaotic arrangement of molecules and don’t have a clear melting point. Instead, they show a glass transition temperature (Tg).
Polymer structure forms the backbone of how these materials behave in real-world applications. Picture polymer chains as incredibly long molecular necklaces, where each bead represents a repeating unit called a monomer. The way these chains arrange themselves determines whether you’re dealing with a rigid plastic bottle or a stretchy rubber band.

Crystalline polymers feature chains that pack together in highly ordered, repeating patterns. Think of soldiers standing in perfect formation – every chain knows exactly where it belongs. This organized arrangement creates distinct regions called crystallites, where molecular chains align parallel to each other in regular, three-dimensional structures.
Amorphous polymers take a completely different approach. Their chains twist and tangle randomly, like a bowl of spaghetti. No organized pattern exists, and the chains adopt whatever position feels comfortable without regard for their neighbors.
Semi-crystalline polymers represent a middle ground, containing both ordered crystalline regions and disordered amorphous areas within the same material. Most commercial polymers fall into this category, with crystallinity levels ranging from 10% to 95%.
The degree of crystallinity directly influences mechanical properties, transparency, and processing behavior. High-density polyethylene (HDPE) achieves 60-80% crystallinity, resulting in excellent strength and chemical resistance. Meanwhile, polystyrene remains largely amorphous, providing clarity and easy processing for food packaging applications.

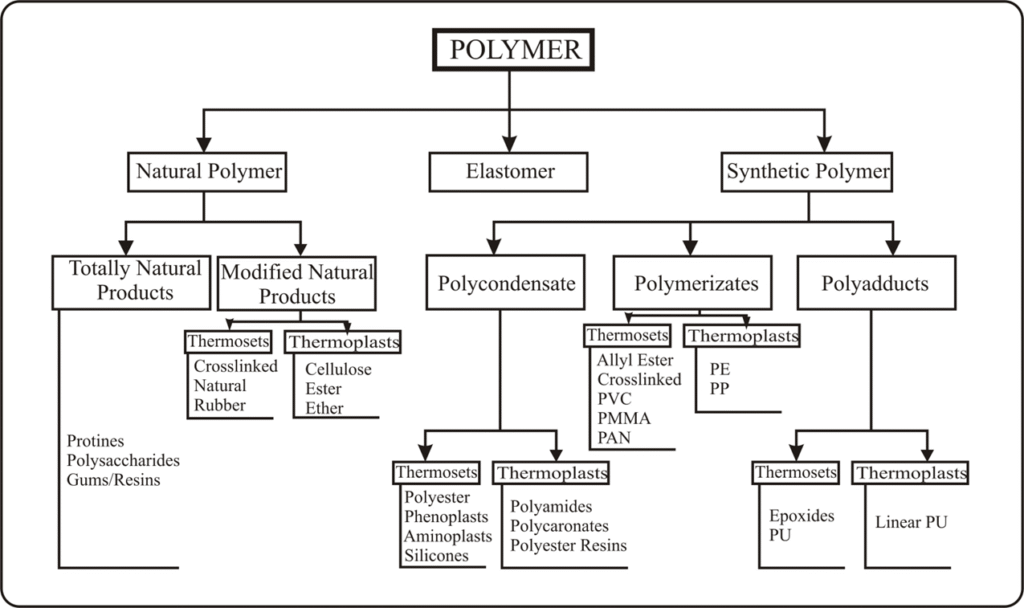

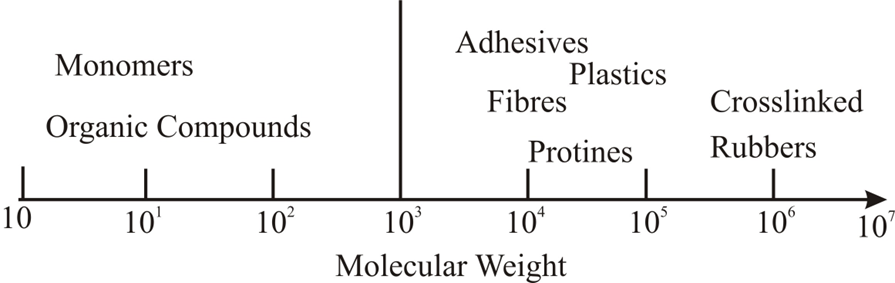
Polymers are a large class of materials consisting of many small molecules (called monomers) that can be linked together to form long chains, thus they are known as macromolecules




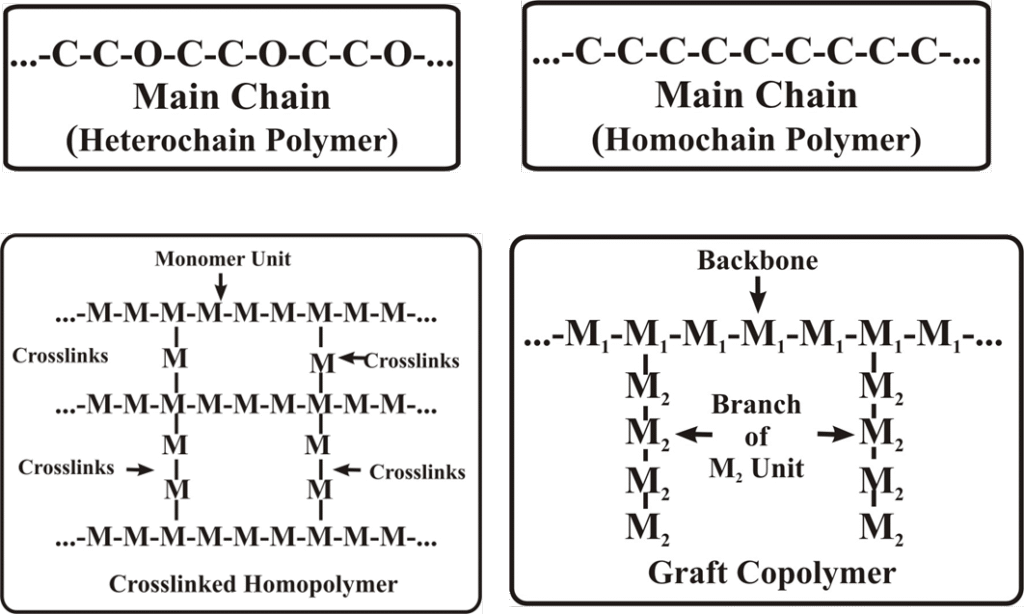
A polymer consist of identical monomers or monomers of different chemical structure and accordingly they are called homopolymer and copolymers respectively. If the main chain is made up of same species of atoms, the polymer is called ‘homochain polymer’ Graft copolymer are branched structures in which the monomer segments on the branches and backbone differ
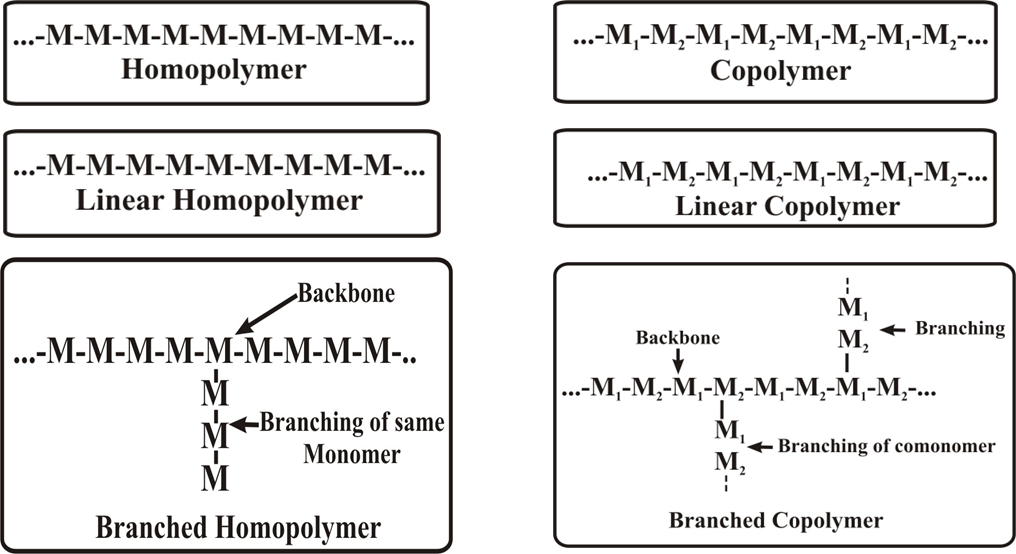
The monomeric unit in a polymer may be present in a linear, branched or cross-linked (three dimensional) structure
TACTICITY–
1. The head to tail configuration in which the functional groups are all on the same side of the chain, is called ‘isotactic polymers’.
2. If the arrangements of functional groups are at random around the main, it is called ‘atactic polymers’ e.g. polypropylene.
3. If the arrangements of side groups is in alternating fashion, it is called ‘synditactic polymers’ e.g. gutta percha.
Thermoplastics Polymers: Thermoplastics are resins that repeatedly soften when heated and harden when cooled
Thermosetting Polymers : Thermosets are resins that undergo chemical changes during processing to become permanently insoluble and infusible due to they formed three-dimensional cross linked network structure when heat is applied.
Difference Between Thermoplastics and Thermosetting Polymers
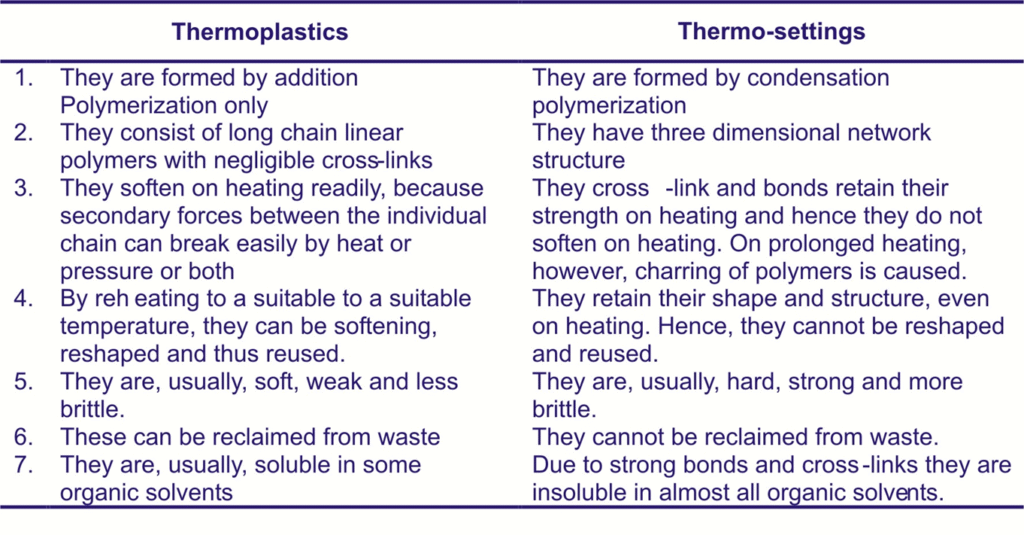

In additions to size of the molecules and their distribution, the shapes or structure, of individual polymer molecules also play an important role in determining the properties and process-ability of plastics.
Because of the geometry, or morphology, of those molecules some can come closer together than other. These are defined as crystalline, all other are as amorphous. Morphology influences such properties as mechanical and thermal, swelling and solubility, specific gravity and chemical and gravity properties.
This behavior of morphology basically occurs with thermoplastics, not thermosetting. When thermosetting are processed, their individual chain segments are strongly together during a chemical reaction that is irreversible.

A small region of a macromolecules materials in which portions of large molecules are linked to each other in some regular way, is called crystalline.
1.Crystallization imparts a denser packing of molecules, thereby increasing the intermolecular forces of attraction.
2.This accounts for a higher and sharper softening point, greater rigidity and strength and greater density. A completely crystalline polymer tends to acquire brittleness.

An ‘amorphous polymers’ is characterized by completely random arrangement of molecules.
While a crystalline region (called crystallites) embedded in amorphous random matrix. Typical crystalline solids are associated with regularly if external form. Polymers with a long repeating unit or with a low degree of symmetry do not crystallize easily and, therefore, generally form amorphous structure e.g. Polystyrene, polyvinyl acetate and polymethyl methacrylate (all are having bulky side groups attached at random to the main carbon chain) are typically amorphous.
An ‘amorphous polymers’ is characterized by completely random arrangement of molecules.

• Relatively short chains organize themselves into crystalline structures more readily than longer molecules. Therefore, the degree of polymerization (DP) is an important factor in determining the crystallinity of a polymer.
• Polymers with a high DP have difficulty organizing into layers because they tend to become tangled.
• As symmetrical molecules approaches within a critical distance, crystal begins to form in the areas where they are most densely packed.
• A crystallized area is stiffer and stronger, a non-crystalline (amorphous) area together and more flexible.
• An increasing crystalline in polypropylene increased in creep, heat and stress cracking as well as increased in mould shrinkage.
•Crystallization tendency decreases by co-polymerization, because it lowers the structural symmetry, so by controlling the extent of co-polymerization, the relative extent of crystallize.
• The amorphous region can be adjusted to get required hardens, rigidity, heat resistance and flexibility.

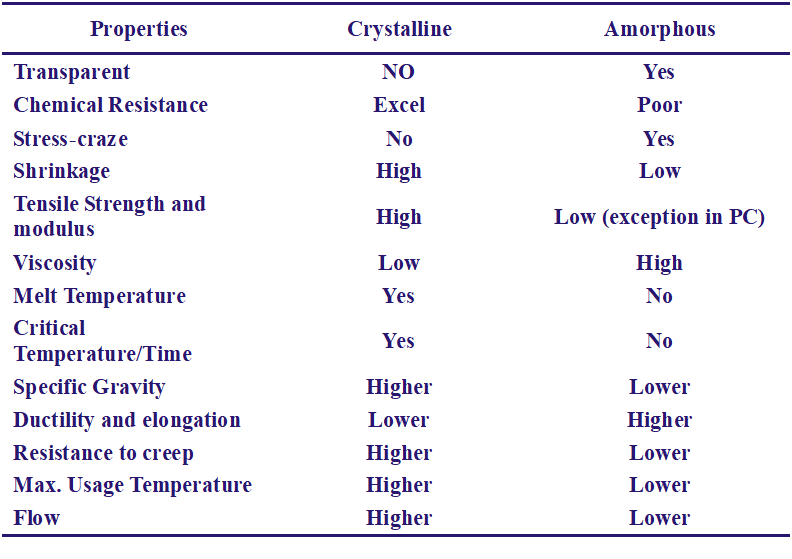
The Distinctive Characteristics of Polymers:
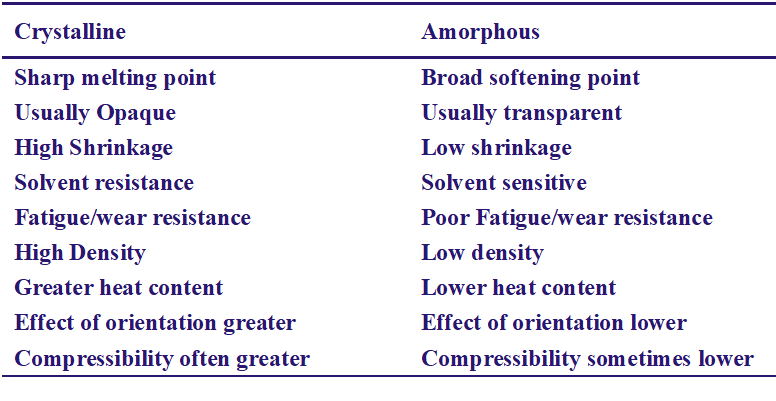
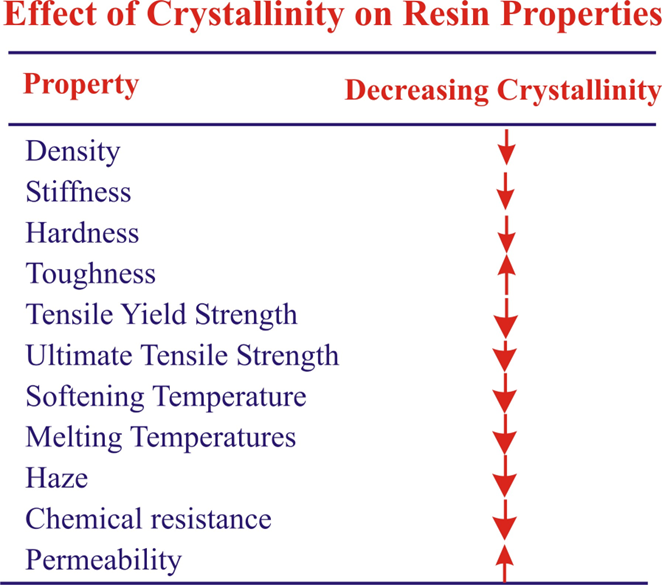
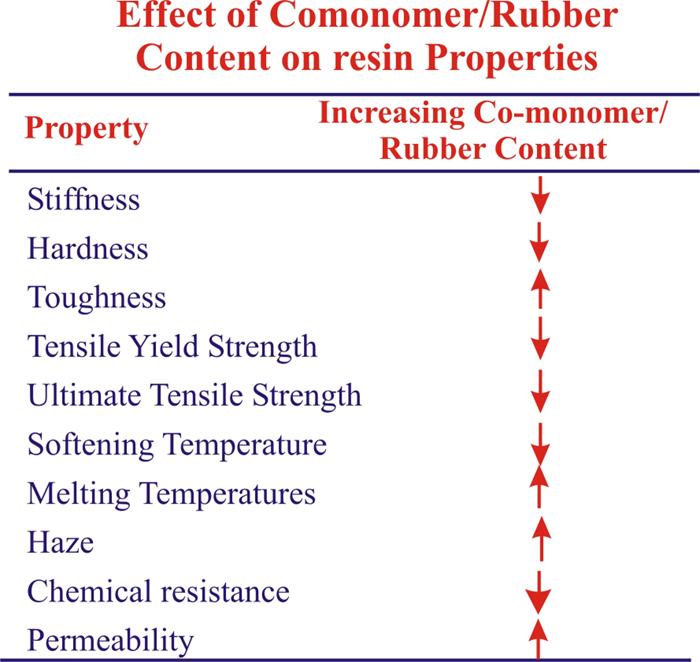
Conclusion :
Both crystalline and amorphous polymers bring unique strengths , and your choice really comes down to what matters most for your project. Crystalline polymers give you that durability and chemical resistance when you need materials that can handle tough conditions, while amorphous polymers shine when you need flexibility and easy processing. Think about packaging films versus impact-resistant safety gear – each application calls for different polymer properties.
The key is matching the polymer structure to your specific requirements rather than assuming one type is automatically better than the other. Look at factors like operating temperature, mechanical stress, processing methods, and cost constraints when making your decision. Take time to test different options if possible, and don’t hesitate to consult with polymer specialists who can help you navigate the technical details. The right polymer choice can make or break your product’s performance, so it’s worth getting this decision right from the start.









Post Comment